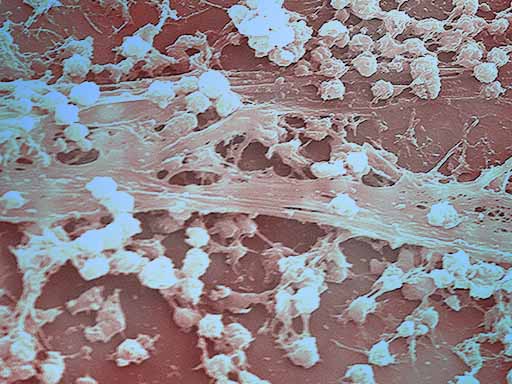
BIOFILM
Nouvex™ is shown to eradicate an established biofilm. “Biofilm forms on most untreated surfaces in moist environments and contributes to degradation, staining, and odors in a multitude of situations,” said Thomas E. Hopkins, Poly Group Chief Scientific Officer. “Biofilms are of particular concern due to the difficulty in gaining access to the bacteria present in order to eradicate. Nouvex represents a new approach to solving problems of microbial degradation of materials as well as the spread of undesirable bacteria.”
“Safely removing biofilm from plastics and other non-food products is a real challenge, and we are excited about the results of this testing,” said Craig Kalmer, Poly Group Chief Operating Officer. “This enhances our market opportunities as part of the first phase in expanding the use of Nouvex.”
The EPA has approved Nouvex N950-1010 Master Batch for use in non-food contact coatings, papers, textiles, and plastics such as thermoset and thermoplastics and synthetics; injection molding; extrusions and powder coatings to control microorganisms that cause deterioration, discoloration, and odor. Nouvex is registered in 49 of 50 states, California is pending.
Nouvex, when tested using EPA required protocols, demonstrates that, when used as directed, is safe to the environment and provides effective material protection as a preservative. The EPA registration is based on independent laboratory testing which proves the ability of Nouvex to be effective in preserving a wide variety of materials.
The antimicrobial market is a multi-billion-dollar industry that presents multiple options and pathways for commercializing Nouvex. Widely published statistics from the Centers for Disease Control and Prevention (CDC) estimate infections acquired in U.S. hospitals affect two million individuals every year and result in nearly 100,000 deaths annually.